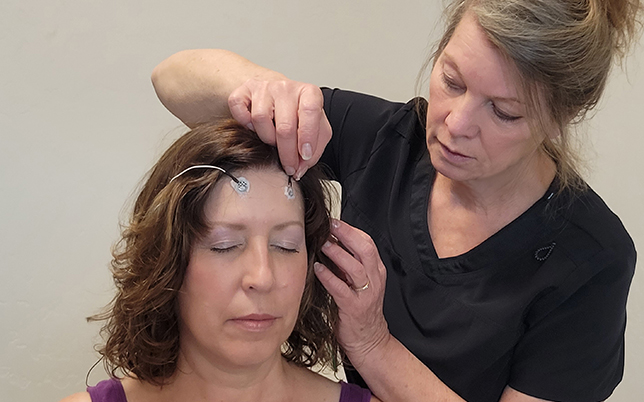
The MicroCurrent Neurofeedback system is science based and safe. The IASIS methodolgy is supported by studies that were conducted across major Universities and research centers. It has been shown to yield life changing results for nearly every patient we treat at The Brain Gym in Rifle, Colorado.
An article posted on Science Daily reports that:
“Using a form of low-impulse electrical stimulation to the brain, documented by neuroimaging, researchers report significantly improved neural function in participants with mild traumatic brain injury (mTBI).”
UC San Diego Health Report
Science Daily Article
A Pilot treatment study for Mild Traumatic Brain Injury
Clinical Trials: Mild traumatic brain injury (mTBI)
The history of MCN
Clinical Efficacy of IASIS Microcurrent Neurofeedback
Brain Gauge Research and Studies
Study from the University of California, San Diego.
The U.S. Code of Federal Regulations (C.F.R.) Section 882.5050 holds as follows:
(a) Identification. A biofeedback device is an instrument that provides a visual or auditory signal corresponding to the status of one or more of a patient’s physiological parameters (e.g., brain alpha wave activity, muscle activity, skin temperature, etc.) so that the patient can control voluntarily these physiological parameters.
(b) Classification. Class II (special controls). The device is exempt from premarket notification procedures in subpart E of part 807 of this chapter when it is a prescription battery powered device that is indicated for relaxation training and muscle reeducation and prescription use, subject to §882.9.
[44 FR 51730, Sept. 4, 1979, as amended at 63 FR 59229, Nov. 3, 1998]